Veterinary diagnosis of Avian Influenza H9 Virus Antibody ELISA Kit
Cat.No.: E604101
Method: ELISA





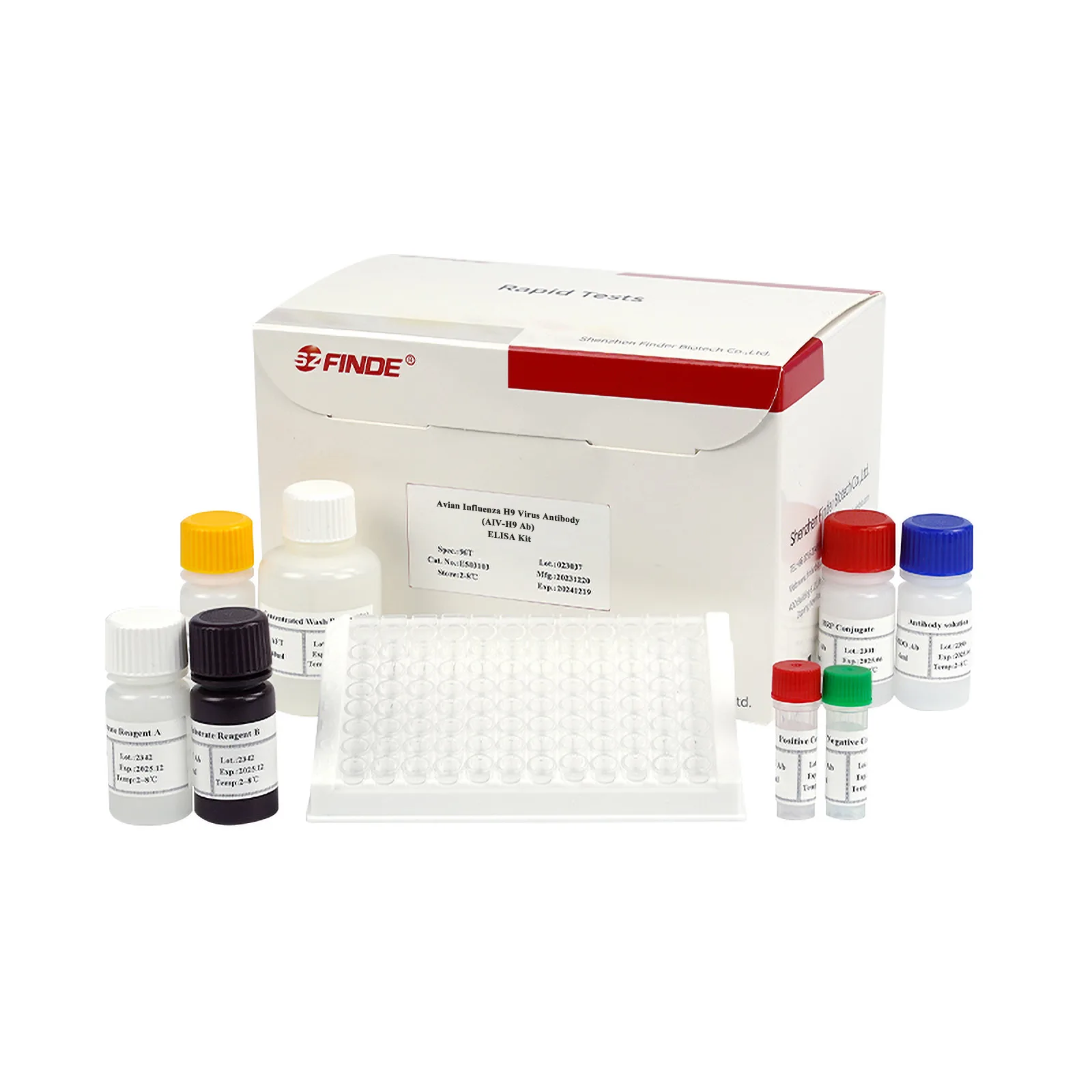
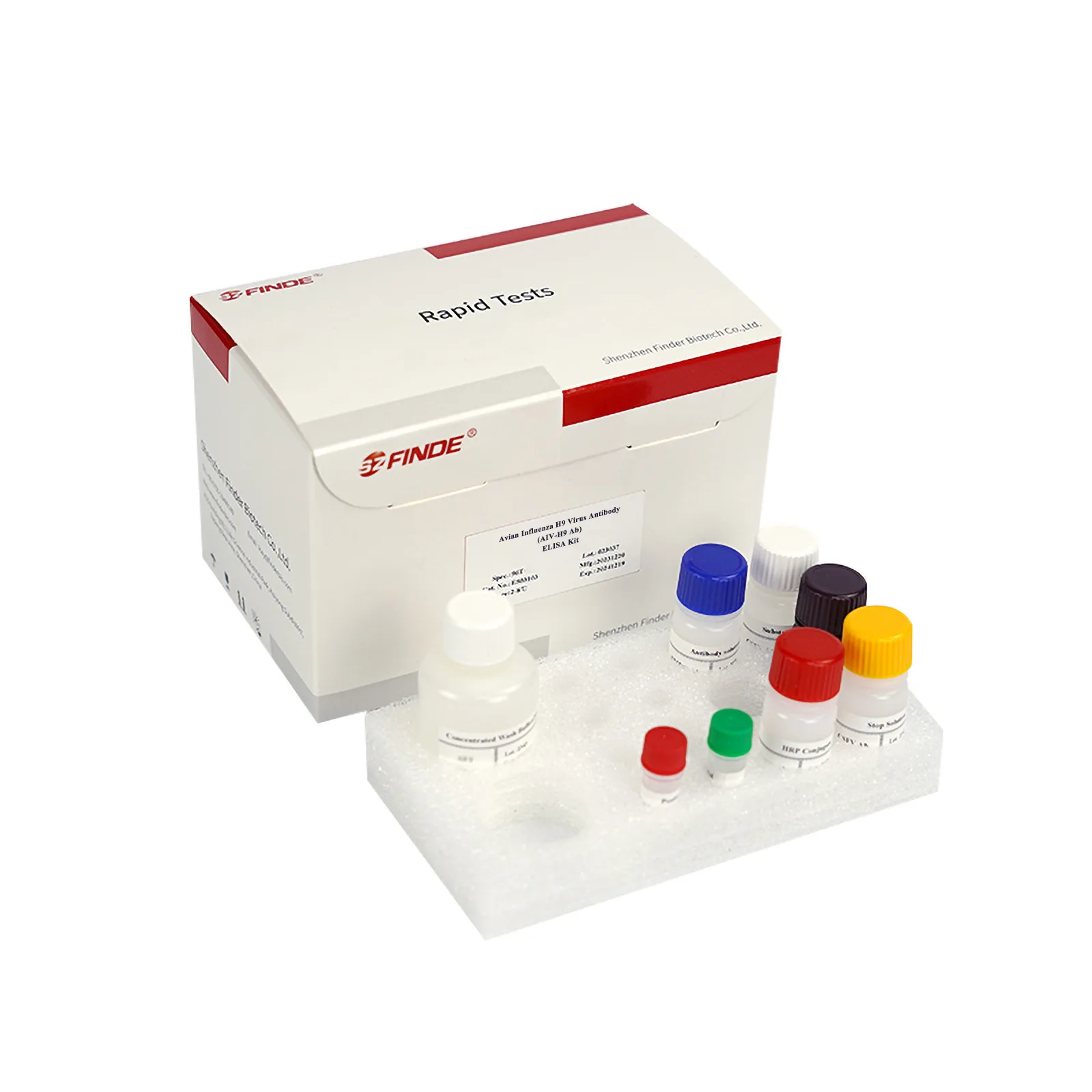


Properties
Model Number
E604101
Product Name
Veterinary diagnosis of Avian Influenza H9 Virus Antibody ELISA Kit
Method
ELISA
Intended Use
Avian Influenza H9 Virus Antibody ELISA Kit is used to detect H9 subtype avian influenza virus antibodies in poultry serum, helping evaluate infection status or vaccine immunization effectiveness.
Test Sample
Serum
Incubation Time
30min+30min+15min
Storage
2-8℃
Shelf Life
12 months
Certificate
ISO9001:2015 & GMP for animal drugs
Specification
96T\96T*2\96T*5
Packing
Foam box with ice bags, packed in carton
Single Package Size
180MM*110MM*115MM\180MM*110MM*115MM\190MM*160MM*145MM
Single Gross Weight
0.40kg\0.55kg\1.42kg
Kit Content
| Reagent | Specification | ||
| Microtiter Plate | 96wells | 96wells×2 | 96wells×5 |
| HRP conjugate (red cap) | 1×11mL | 2×11mL | 2×26mL |
| Antibody solution (blue cap) | 1×6mL | 1×11mL | 1×26mL |
| Concentrated Wash Buffer (20×) (white cap) | 1×40mL | 1×40mL | 1×200mL |
| Substrate Reagent A
(white cap) |
1×6mL | 1×11mL | 1×26mL |
| Substrate Reagent B
(black cap) |
1×6mL | 1×11mL
(brown cap) |
1×26mL
|
| Stop Solution (yellow cap) | 1×6mL | 1×11mL | 1×26mL |
| Positive Control (red cap) | 1×1.0mL | 1×1.5mL | 1×2.0mL |
| Negative Control (green cap) | 1×1.0mL | 1×1.5mL | 1×2.0mL |
| Adhesive Membrane | 1 | 2 | 5 |
| Sealed bag | 1 | 1 | 2 |
| Instructions | 1 | 1 | 1 |
Product Details
Overview of the kit
The Avian Influenza H9 ELISA kit is primarily used to detect antibodies against the H9 subtype of avian influenza virus in poultry. Therefore, it provides rapid and accurate results, offering valuable data for disease control and avian influenza monitoring.
Overview of Avian Influenza H9 Virus
H9 avian influenza is a subtype that primarily affects poultry, including chickens and ducks. Consequently, this virus can spread rapidly and cause significant economic loss in poultry farming. Thus, early detection is critical to control outbreaks and prevent further transmission.
Features of the Avian Influenza H9 Antibody ELISA Kit
- High Sensitivity
The kit detects even low concentrations of H9 virus antibodies, therefore ensuring accurate results. - Rapid Detection
The test is simple and fast, with results available in approximately 30 minutes. As a result, it is ideal for on-site testing. - High Specificity
It specifically targets H9 antibodies, which ensures precise detection without interference from other antibodies. - Good Stability
The kit maintains stability under various storage and usage conditions.
Advantages of the Avian Influenza H9 Antibody ELISA Kit
- Easy to Use
No specialized training is required, which makes the kit accessible for general use. - Efficient and Cost-effective
The kit delivers quick, accurate results, therefore reducing labor costs and improving overall efficiency. - Wide Application
It is suitable for use in poultry farms, veterinary testing, and epidemiological monitoring. - International Standards
The kit meets global quarantine and food safety standards, thus ensuring its applicability worldwide.
Application Fields
- Avian Influenza Surveillance
It plays a key role in monitoring and controlling the spread of H9 avian influenza. As a result, it ensures timely detection and response. - Poultry Farming
Widely used in poultry farms to screen for avian influenza, which helps mitigate disease risks and ensures farm safety. - Veterinary Testing
Veterinarians use the kit to monitor poultry health and detect infections early. - Food Safety Testing
The kit is used to ensure that poultry meat products are free from avian influenza, therefore safeguarding public health.
Conclusion
In conclusion, the Avian Influenza H9 Antibody ELISA Kit offers an efficient, sensitive, and cost-effective method for detecting the virus. Additionally, it is widely used in surveillance, farming, veterinary checks, and food safety testing. Therefore, early detection allows for timely intervention, reducing the virus’s spread and protecting both animal health and food safety.
Test Procedure
-jpg.webp)
Certficates

How To Use?